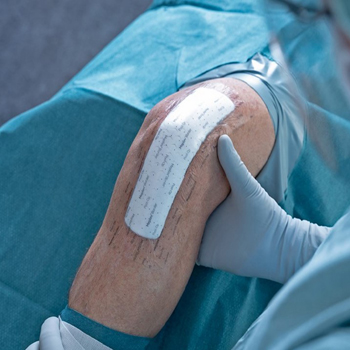
Mepilex Border Post-Op
Mepilex Border Post-Op je obloga »vse v enem« za oskrbo kirurških ran, ki učinkovito vpije in v sebi zadrži izločke iz kirurške rane. Sloj mehkega silikona Safetac zmanjšuje pojavnost mehurjev1 in zagotavlja menjavo obloge brez poškodbe rane ali kože v njeni okolici, hkrati pa zmanjšuje bolečino pri prevezi2-4. Oblogo lahko privzdignemo in preverimo stanje rane ter jo ponovno namestimo na rano, pri tem pa ne tvegamo, da bi se lepljivost obloge zmanjšala. Mepilex Border Post-Op je lahko nameščen do 14 dni8 zapored, odvisno od stanja rane in od kože v njeni okolici ter od sprejete klinične prakse. Hkrati Safetac zatesni robove rane in tako preprečuje iztekanje izločka na kožo v okolici rane. S tem preprečuje nastanek maceracije5,6,7. Na koži ne pušča ostankov in kože ne draži, je hipoalergena. Zahvaljujoč tehnologiji s patentiranim vzorcem zarez omogoča optimalno prilagajanje zahtevnim oblikam telesa ter tako povečuje mobilnost pacienta2-4. Široki prosojni robovi omogočajo lažji nadzor okolice rane. Zmanjšuje tveganje za nastanek raztrganine ter tveganje oprijemanja šivov in zank za zapiranje ran. Zaščitni poliuretanski film predstavlja pregrado pred virusi in bakterijami večjimi od 25nm in omogoča tuširanje. Poleg tega predstavljajo prednost manjši stroški3,4 zaradi manjšega števila menjav in manjše porabe dodatnega materiala.Področja uporabe
Obloga Mepilex Border Post-Op je namenjena za oskrbo akutnih ran, kot so na primer kirurške rane, vreznine ali odrgnine. Je posebej oblikovana za uporabo po operaciji in namenjena za vpijanje krvi. Obloga je visoko upogljiva in je idealna za oskrbo sklepov kot so boki, kolena ali komolci9-11.
Vsaka dimenzija obloge je optimizirana za svoj namen glede na vpojnost, upogljivost in potrebo po dolgotrajni namestitvi. Dimenzije 20 cm in večje so namenjene ranam z zelo veliko izločka, manjše dimenzije pa takim z veliko ali srednje veliko izločka. Ne potrebuje sekundarne pritrditve.
Opomba: uporaba obloge Mepilex Border Heel kot del profilaktične terapije ne izključuje potrebe po upoštevanju in izvajanju celovitega kliničnega protokola za preprečevanje razjed zaradi pritiska!

|
||||
| Šifra | Naziv artikla | Pakiranje kos/zavitek | NENSI šifra | |
| 496100 | Mepilex Border Post Op 6x8cm | 10 | 1060330 | |
| 496200 | Mepilex Border Post Op 9x10cm | 10 | ||
| 496300 | Mepilex Border Post Op 10x15cm | 10 | ||
| 496400 | Mepilex Border Post Op 10x20cm | 10 | ||
| 496450 | Mepilex Border Post Op 10x25cm | 10 | 1060346 | |
| 496600 | Mepilex Border Post Op 10x30cm | 10 | ||
| 496650 | Mepilex Border Post Op 10x35cm | 5 | ||
Reference:
1. Johansson C. et al. An assessment of a self-adherent, soft silicone dressing in post operative wound care following hip and knee arthroplasty. Poster presentation at EWMA, Brussels, Belgium 2012.
2. Beele H. et al. A prospective randomized controlled clinical investigation comparing two post-operative wound dressings used after elective hip and knee replacement; Mepilex® Border Post-Op versus Aquacel® Surgical. International Journal of Orthopaedic and Trauma Nursing, 2020.
3. Zarghooni K, et al. Is the use of modern versus conventional wound dressings warranted after primary knee and hip arthroplasty? Acta Orthop Belg. 2015;81(4):768-775.
4. Bredow J. et al. Evaluation of Absorbent Versus Conventional Wound Dressing. A Randomized Controlled Study in Orthopedic Surgery. Deutsche Arzteblatt Intternational, 2018.
5. Meaume S. et al. A study to compare a new self adherent soft silicone dressing with aself adherent polymer dressing in stage II pressure ulcers. Ostomy Wound Management, 2003.
6. Feili F et al. Retention capacity. Poster presentation at the EWMA conference, Lisbon, Portugal 2008.
7. Wiberg A.B. et al. Preventing maceration with a soft silicone dressing: in-vitro evaluations. Poster presented at the 3rd Congress of the WUWHS, Toronto, Canada, 2008.
8. Van Overschelde P, et al. Undisturbed wound healing: a single centre retrospective study investigating patient reported outcomes and clinical validity of extended dressing wear time for incisional healing following orthopaedic surgery, the ARCTIS study. Journal of Wound Care, 2024.
9. Bredow J, et al. Randomized clinical trial to evaluate the performance of a flexible self-adherent absorbent dressing coated with a soft silicone layer after hip, knee or spinal surgery in comparison to standard wound dressing. Poster presentation at the 5th Congress of WUWHS, Florence, Italy, 25-29 Sep 2016.
10. Zarghooni K, et al. Effect of a modern dressing compared to standard dressings on outcome after primary hip and knee arthroplasty: a prospective, non-randomised controlled study. E-poster presentation at EWMA Conference. London, UK, 13-15 May 2015.
11. Van Overschelde P, et al. A randomised controlled trial comparing two wound dressings used after elective hip and knee arthroplasty. Poster presentation at the 5th Congress of WUWHS, Florence, Italy, 25-29 Sep, 2016.