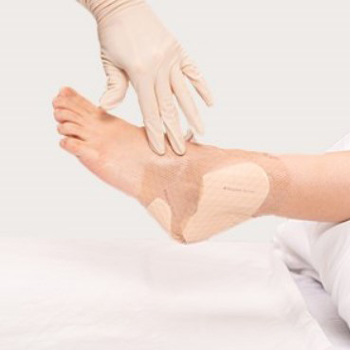
Mepilex Border Heel
Mepilex Border Heel je 5 – slojna obloga s peno »vse v enem«, prilagojena za oskrbo ran na peti4, za zaščito pete in njene okolice, Ahilove tetive ter medialno in lateralno pokritost gležnja. Obloga je 5 – slojna, ima rob in je po celotni površini prekrita s slojem mehkega silikona Safetac. Ko se uporablja kot preventiva, dokazano pripomore k zaščiti kože pred nastankom razjed zaradi pritiska, striga in trenja1-3,10,11. Silikonska plast s tehnologijo Safetac ščiti kožo tako, da ohranja optimalni položaj in omejuje pritisk, strig in trenje med oblogo in predelom teles3,4,8. Kadar se Mepilex Border Heel uporablja za oskrbo ran, učinkovito absorbira in zadržuje izločke4-6,12 ter uravnava vlažnost, s čimer preprečuje tveganje za nastanek maceracije6,7. Z ohranjanjem vlažnega okolja podpira odstranjevanje odmrlih tkiv, zato lahko opazite začetno povečanje velikosti rane. To je običajno in pričakovano. Safetac sloj mehkega silikona zatesni robove rane in tako preprečuje iztekanje izločka na kožo v okolico rane. Prav tako Safetac sloj mehkega silikona zagotavlja odstranjevanje obloge brez travme oz. brez poškodb tkiva v rani8 in v njeni okolici in hkrati brez bolečin za pacienta9,14. Manj bolečin pomeni nižjo raven stresa pri pacientih, kar posledično pozitivno vpliva tudi na proces celjenja rane. Vgrajeni posebni zavihki in debelejši robovi pa olajšajo njeno nameščanje ter odstranjevanje13. Obloga lahko na rani ostane nameščena več dni, odvisno od stanja rane in količine izločka ter sprejete klinične prakse. Oblogo lahko tudi privzdignemo in preverimo stanje kože ali rane ter jo ponovno namestimo, pri tem pa ne tvegamo, da bi se lepljivost obloge zmanjšala. V veliko pomoč pri zdravstveni negi, beleženju količine/širjenja izločka in načrtovanju menjave obloge je kazalnik obsega izločka13. Ne potrebuje sekundarne pritrditve in omogočajo prhanje. Safetac sloj mehkega silikona zatesni robove rane in tako preprečuje iztekanje izločka na kožo v okolico rane. Prav tako Safetac sloj mehkega silikona zagotavlja odstranjevanje obloge brez travme oz. brez poškodb tkiva v rani5 in v njeni okolici in hkrati brez bolečin za pacienta11,12. Manj bolečin pomeni nižjo raven stresa pri pacientih, kar posledično pozitivno vpliva tudi na proces celjenja rane. Vgrajeni posebni zavihki in debelejši robovi pa olajšajo njeno nameščanje ter odstranjevanje. Obloga lahko na rani ostane nameščena več dni, odvisno od stanja rane in količine izločka ter sprejete klinične prakse. Oblogo lahko tudi privzdignemo in preverimo stanje rane ter jo ponovno namestimo na rano, pri tem pa ne tvegamo, da bi se lepljivost obloge zmanjšala. V veliko pomoč pri zdravstveni negi, beleženju količine/širjenja izločka in načrtovanju menjave obloge13 je kazalnik obsega izločka. Ne potrebuje sekundarne pritrditve in omogočajo prhanje4.Področja uporabe
Obloga je primerna za uporabo pri ogroženih pacientih10, kot so nepokretni, tisti s slabo prekrvavitvijo kože in poškodovano kožo, v urgentnih sobah, v intenzivni negi ali pred in med dolgotrajnimi kirurškimi posegi.
Prilagojena je za preprečevanje poškodb kože oz. nastanka razjed zaradi pritiska in za oskrbo odprtih ran z izcedki na petah, vključno s preležaninami, ranami zaradi diabetesa, razjedami pete, travmatskimi ranami in drugimi sekundarnimi ranami.
Opomba: uporaba obloge Mepilex Border Heel kot del profilaktične terapije ne izključuje potrebe po upoštevanju in izvajanju celovitega kliničnega protokola za preprečevanje razjed zaradi pritiska!

|
||||
| Šifra | Naziv artikla | Pakiranje kos/zavitek | NENSI šifra | |
| 282750 | Mepilex Border Heel 22x23cm | 6 | 1060316 | |
Reference:
1. Santamaria N, et al. Clinical effectiveness of a silicone foam dressing for the prevention of heel pressure ulcers in critically ill patients: Border II Trial. J Wound Care. 2015;24(8):340-345.
2. Hahnel, E., El Genedy, M., Tomova-Simitchieva, T., Hauß, A., Stroux,A., Lechner,A., Richter,C., AkdeniziD,M., Blume-Peytavi, U., Löber, N. and Kottner, J. The effectiveness of two silicone dressings for sacral and heel pressure ulcer prevention compared with no dressings in high-risk intensive care unit patients: a randomized controlled parallel-group trial, British Journal of Dermatology, 2019, https://doi.org/10.1111/bjd.18621
3. Levy A, et al. The biomechanical efficacy of dressings in preventing heel ulcers. J Tissue Viability. 2015;24(1) :1-11.
4. Molnlycke Health Care. Verification test of new heel shape. Report no. 20170221-007. 21 February 2017. Data on file. Mepilex Border Heel
5. Mölnlycke Health Care. Data on file. 2000
6. Molnlycke Health Care. Design verification PUP16. Report no. 20170515-002. 15 May 2017. Data on file. Mepilex Border Sacrum
7. Woo K, Coutts P.M., Price P, Harding K, Sibbald R.G. A randomised crossover investigation of pain at dressing change comparing 2 foam dressings Advances in Skin and Wound Care 2009;22(7):304-310.
8. Meaume, S., Van De Looverbosch, D., Heyman, H., Romanelli, M., Ciangherotti, A., Charpin, S. S. A study to compare a new self-adherent soft silicone dressing with a self-adherent polymer dressing in stage II pressure ulcers. Ostomy Wound Management 2003;49(9):44-52.
9. White, R. A multinational survey of the assessment of pain when removing dressings. Wounds UK 2008;4:14-22. Available from: http://www.wounds-uk.com/journal-articles/a-multinational-survey-of-the-assessment-of-pain-when-removing-dressings-1 [Accessed 4 February 2019]. Mepilex Border (and other dressings with Safetac) vs. dressings with traditional adhesives (adhesive foams, hydrocolloids etc.)
10. Tayyib N, et al. Effectiveness of pressure ulcer prevention strategies for adult patients in intensive care units: a systematic review. Worldviews Evid Based Nurs 2016;13(6):432-444.
11. World Union of Wound Healing Societies (WUWHS). Consensus Document: Role of dressings in pressure ulcer prevention. London, UK: Wounds Int; 2016.
12. Molnlycke Health Care. External test Lab report id SMTL 00/1278/01,00/1235/01, 21 September 2000. Data on file. Mepilex Border
13. Davies, P. User evaluation of interface dressings for pressure ulcer prevention. Mölnlycke Health Care (GMCS-2017-058). 18 April 2017. Data on file.
14. White R. A multinational survey of the assessment of pain when removing dressings. Wounds UK. 4 (1). 2008.